Medical devices and associated systems are especially vulnerable.
Medical device cybersecurity risk mitigation conference.
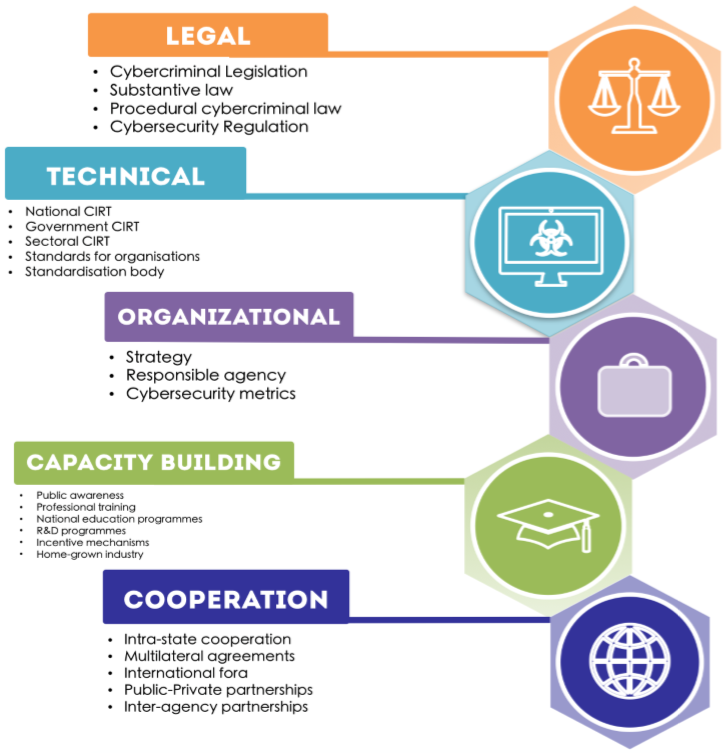
Medical device security will not be solved individually.
Ensuring compliance with regulatory requirements and industry standards to mitigate cyber risks and continuously improve medical device security in addition to collaborating and sharing responsibility between health delivery organizations and manufacturers.
Nova leah specialize in expert cybersecurity risk management software solutions for the medical device industry.
Medical device manufacturers mdms and health care delivery organizations hdos should take steps to ensure appropriate safeguards are in place.
Why is the medical device cybersecurity risk mitigation conference important to medical device security and manufacturing teams.
Healthcare is an ecosystem that medical devices play a role in.
Managing cybersecurity risk through various assessments robust management systems.
This year s medical device cybersecurity risk mitigation conference will virtually connect security leaders to share best practices in the following modules.
5th annual medical device cybersecurity risk mitigation conference.
4th annual medical device cybersecurity risk mitigation conference july 23 24 2019 arlington va.
9 30 initiatives to further medical device cybersecurity mitre is developing a rubric for the common vulnerability scoring system cvss to help medical device manufacturers mdms and healthcare delivery organizations hdos more.
Cybersecurity collaboration between mdms hdos to ensure patient safety module 3.
4th annual medical device cybersecurity risk mitigation conference july 23 24 2019 arlington va.
Security in the healthcare industry is in a higher risk state than almost any other industry.
This year s medical device cybersecurity risk mitigation conference will virtually connect security leaders to share best practices in the following modules.
Selectevidence supports manufacturers in designing verifying and certifying medical devices to meet fda cybersecurity recommendations best practices for connected medical devices and industry security standards.
Healthcare and manufacturing are coming together to solve these challenges and this conference.
Cybersecurity collaboration between mdms hdos to ensure patient safety.
2019 medical device security 101 conference.
Mitigation strategies for cybersecurity vulnerabilities in legacy products hdos utilize medical equipment developed 10 to 20 years ago when cybersecurity was not considered a fundamental factor of product development and the industry is continuously working to evaluate the cyber risk of each device model in order to patch.
Managing cybersecurity risk through various assessments robust management systems module 2.